

30-32 The group contributions for a,co-amino acids are considerably smaller than the contributions reported for the same groups in the case of a-amino acids.
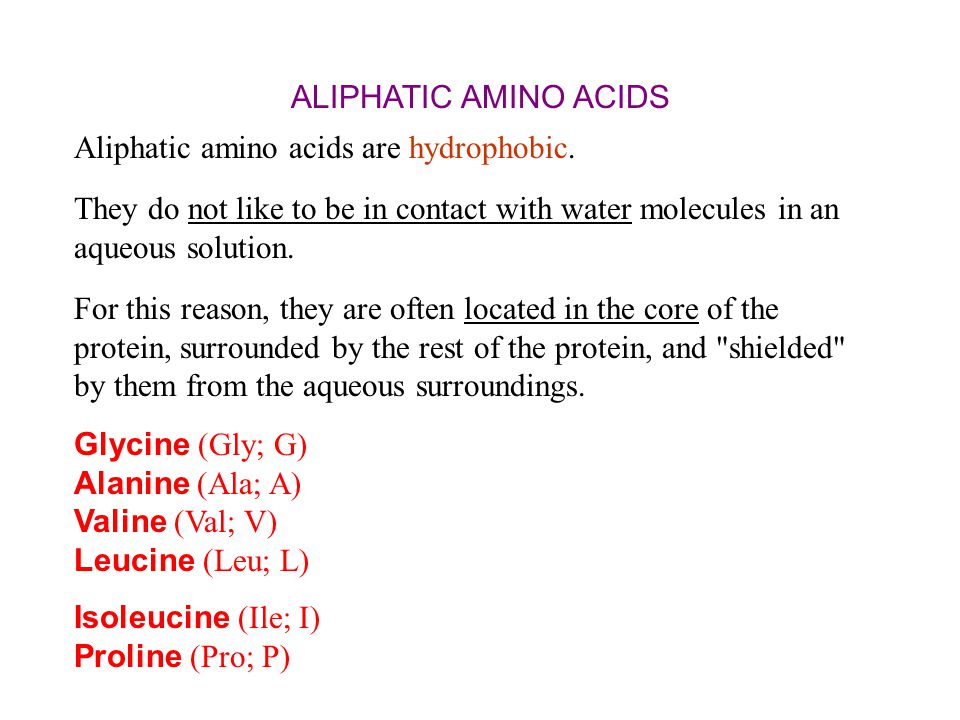
Hydrophobic amino acids in aqueous solutions series#
The group contributions for a-amino acids at 298.15 К are similar to the volumetric contributions corresponding to other homologous series such as alcohols, amides, and other model solutes. TABLE 4.1 Volumetric Contributions of the Methylene, Amnio, and Carboxylic Groups to the Partial Molar Volume of a-Amino Acids and a,со-Amino Acids. Table 4.1 presents a comparison of the volumetric contributions of the methylene group and the polar carboxylic and amino groups in water determined in previous works at several temperatures. 17-28įIGURE 4.1 Comparison of the partial molar volumes at infinite dilution of a-amino acids and a,co-amino acids as a function of the number of methylene groups.
This suggests that solute-solvent polar interactions are higher for a,co-amino acids. The volumetric properties of amino acids in aqueous solutions show that the partial molar volumes at infinite dilution of a,co-amino acids in aqueous solutions are smaller than the corresponding values for a-amino acids at the same temperatures. The dependence of the partial molar volumes at infinite dilution of a-amino acids and a,co-amino acids with the number of methylene groups is well represented by linear equations indicating that the volumetric contribution of the methylene group is additive. Both series of compounds follow a similar behavior.

The behavior of the partial molar volumes at infinite dilution of a-amino acids and a,co-amino acids as a function of the number of methylene groups is presented in Figure 4.1. hi this equation, a and b are empirical constants and Vf is the partial molar volume at infinite dilution. Concentration is expressed in molality, m, and the experimental data usually are fitted to second-order polynomial equations as it is shown by eq (4.2). The dependence of the apparent molar volumes of the amino acids with the concentration at the working temperatures is fitted by least squares method to obtain an equation that describes the behavior of the apparent molar volumes of the amino acids. Where M is the molar mass of the solute, m is the molal concentration of the aqueous solution, p o is the density of the solvent, and p is the solution density. The apparent molar volume, y, can be calculated from density data using eq (4.1): The last method is one of the more precise methods and it is frequently used. The partial and the apparent molar volumes of the amino acids in solution can be determined from density measurements using different techniques such as picnometry, magnetic float densimetry, and vibrating tube densim- etiy.


 0 kommentar(er)
0 kommentar(er)
